Introduction
Imagine a world without the complicated mechanisms that prevent us from bleeding uncontrollably when we accidentally cut ourselves. That’s where hemostasis comes into play, a vital physiological process that often gets confused with homeostasis but serves an entirely different purpose. Hemostasis is the body’s natural way of stopping bleeding, and it’s a marvel of our physiological design. It’s most effective in dealing with injuries to small blood vessels like arterioles, capillaries, and venules, which are the usual culprits in everyday bleeding episodes. However, it’s ineffective when controlling bleeding from medium or large arteries.When a blood vessel is damaged, its immediate response is to constrict. While the exact mechanism behind this isn’t entirely understood, it likely involves changes in local vasodilator (blood vessel widening) and constrictor (blood vessel narrowing) substances released by endothelial cells and blood cells. This rapid constriction slows down the blood flow in the injured area. Furthermore, the vessel’s constriction presses the opposing endothelial surfaces together, inducing stickiness that keeps them essentially “glued” together. The process of hemostasis occurs in two steps:
1. Formation of a Platelet Plug: The first step involves the formation of a platelet plug. Platelets play a crucial role in this process. When a blood vessel is injured, platelets rush to the scene and start sticking together, forming a plug. This plug temporarily seals the breach, further reducing blood loss.
2. Blood Coagulation (Clotting): The second step is blood coagulation, commonly known as clotting. This complex cascade of reactions involves various clotting factors and proteins working together to create a mesh-like structure within the platelet plug. This mesh traps red blood cells, creating a more stable and permanent seal over the injured vessel.
Formation of a Platelet Plug
Adhesion
The protective endothelium lining is disrupted when an injured blood vessel reveals collagen fibres beneath. It is at this point that platelets come into play. Platelets adhere to collagen with the assistance of Von Willebrand factor, a plasma protein secreted by endothelial cells and platelets themselves. Von Willebrand factor binds to exposed collagen, undergoes a conformational change, and becomes capable of binding to platelets. This interaction effectively bridges the damaged vessel wall and the platelets.
Platelet Activation
Upon binding to collagen, platelets release the contents of their secretory vesicles. These vesicles contain many chemical agents, including adenosine diphosphate (ADP) and serotonin, which act locally to induce changes in platelet metabolism, shape, and surface proteins—a process known as platelet activation. This activation changes the platelets and prompts new platelets to adhere to the previously activated ones, a positive feedback mechanism called platelet aggregation. This rapid accumulation of platelets creates the foundation for the platelet plug within the vessel.
Secretory vesicle contents do not solely drive platelet activation. Adhesion of platelets also triggers the synthesis of thromboxane A2, a member of the eicosanoid family, from arachidonic acid in the platelet plasma membrane. Thromboxane A2 is released into the surrounding fluid, where it further stimulates platelet aggregation and promotes the release of secretory vesicle contents, amplifying the formation of the platelet plug. Another critical contributor to platelet aggregation is fibrinogen, a plasma protein. Fibrinogen forms bridges between aggregating platelets, reinforcing their bond and solidifying the platelet plug’s structure. As the platelet plug matures, it gains additional strength through a remarkable property of platelets—contraction. Platelets contain a high concentration of actin and myosin, proteins responsible for muscle contraction. In the context of hemostasis, these proteins stimulate contraction in aggregated platelets, causing compression and reinforcing the platelet plug.
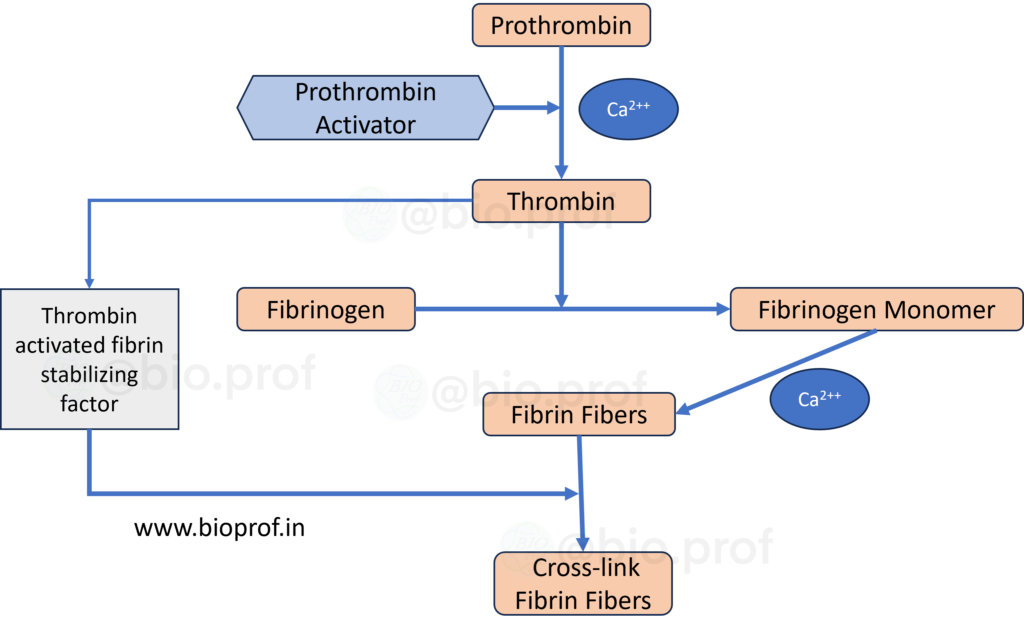
Blood clotting
When a blood vessel sustains an injury, the body initiates a crucial process known as blood clotting or coagulation to staunch the bleeding. At the injury site, blood platelets disintegrate and release a vital phospholipid called thromboplastin, which is also released by the injured tissues. This event triggers a complex cascade of chemical reactions in response to vessel rupture or blood damage. Ultimately, these reactions form a multifaceted group of activated substances known as prothrombin activators. This prothrombin activator catalyses the conversion of prothrombin into thrombin, a pivotal enzyme responsible for transforming fibrinogen into resilient fibrin fibers. These fibrin fibers weave together platelets, blood cells, and plasma to create the essential clot. The rate-limiting factor in blood coagulation lies in the formation of prothrombin activator, as the final stages of clot formation typically progress rapidly. Under normal circumstances, the clotting process takes approximately 2 to 8 minutes. Prothrombin activators formation follows two primary pathways: the extrinsic pathway, initiated by trauma to the vascular wall and surrounding tissues, and the intrinsic pathway, which begins within the blood. Together, these pathways orchestrate a remarkable and essential response to injury, preventing excessive bleeding and promoting the body’s natural healing.
- Prothrombin is a plasma protein with a molecular weight of 68,700.
- Normally present in plasma at around 15 mg/dl.
- It can easily split into smaller compounds, including thrombin (33,700 molecular weight).
- Prothrombin is produced by the liver and requires vitamin K for its normal formation.
- Early stages of fibrin formation involve weak hydrogen bonds, resulting in a weak clot.
- Over time, the fibrin reticulum strengthens with the help of fibrin-stabilizing factor.
- Thrombin activates the fibrin-stabilizing factor.
- This activated substance acts as an enzyme to create covalent bonds and cross-linkages, strengthening the fibrin meshwork.
Extrinsic pathway
In the extrinsic pathway of blood clotting, when tissue is injured, it releases tissue thromboplastin. This thromboplastin then forms a complex with blood coagulation Factor VII. In the presence of calcium ions, this complex enzymatically activates Factor X, transforming it into activated Factor X (Xa). Almost immediately, activated Factor X combines phospholipids released from platelets and Factor V to create the prothrombin activator complex. In the presence of calcium ions (Ca++), this complex efficiently converts prothrombin into thrombin, setting the stage for the clotting process. As clotting commences and thrombin is generated, it triggers the proteolytic activation of Factor V, further accelerating the activation of prothrombin and contributing to the intricate orchestration of the clotting cascade.

intrinsic pathway
Within the intricate tapestry of blood coagulation, the intrinsic pathway represents the second mechanism that comes into play when blood encounters trauma or gets exposed to collagen. The narrative unfolds as blood trauma triggers the activation of Factor XII, accompanied by the release of platelet phospholipids. Upon encountering collagen or a surface like glass, Factor XII undergoes a transformative process, assuming a new molecular identity as “activated Factor XII,” a proteolytic enzyme. In this tumultuous scenario, platelet factor 3 is also set free. The cascade continues with activated Factor XII, subsequently activating Factor XI, a process facilitated by the presence of high molecular weight kininogen and the assistance of prekallikrein. The story gains momentum as activated Factor XI takes its enzymatic prowess to Factor IX, initiating the activation of the latter. In this orchestration, activated Factor IX, alongside activated Factor VIII and platelet factor 3 contributed by the stressed platelets, takes centre stage to activate Factor X. It’s worth noting that Factor VIII, often referred to as antihemophilic factor, is missing in classic haemophilia. Activated Factor X combines Factor V and platelet or tissue phospholipids to form the prothrombin activator complex. As the narrative unfolds, the final act sees the formation of serum, a pale-yellow fluid devoid of fibrinogen and most other clotting factors, marking the aftermath of the blood’s coagulative journey.

| Clotting factor | Synonyms |
| I | Fibrinogen |
| II | Prothrombin |
| III | Thromboplastin |
| IV | Calcium |
| V | Proaccelerin, labile factor, accelerator globin (ACG) |
| VI | Proconvertin, Serum prothrombin conversion accelerator (SPCA), stable factor |
| VII | Antihaemophilic factor (AHF), antihaemophilic factor A, Antihaemophilic globulin (AHG |
| VIII | Plasma thromboplastin component (PTC); Christmas factor, Antihaemophilic factor B |
| IX | Stuart-Prower factor |
| X | Plasma thromboplastin antecedent (PTA), antihaemophilic factor C |
| XI | Hageman factor, glass factor |
| XII | Fibrin-stabilizing factor (FSF), Laki-Lorand facto |
