For other theory about orgin of life, Please visite this blog postThe concept of life’s emergence from inanimate matter has fascinated scientists for centuries. One prominent theory that attempts to unravel this enigma is the Chemical Theory of the Origin of Life, which posits that life arose through a sequence of chemical reactions on the primitive Earth. This revolutionary idea was first proposed by Ernst Haeckel and further developed by Russian biochemist A.I. Oparin and English biologist J.B.S. Haldane in the early 20th century.
The Genesis of the Theory
In 1924, A.I. Oparin introduced the chemical theory by outlining his ideas in “The Origin of Life.” Independently, J.B.S. Haldane contributed to the theory in 1928. This theory, also known as the proteinogenesis theory, suggests that life didn’t spontaneously appear but gradually emerged from inanimate matter through a series of complex chemical reactions on Earth’s primordial landscape.
The Essential Components
The chemical theory proposes four fundamental prerequisites for life’s emergence:
Reducing Atmosphere: The early Earth lacked the oxygen-rich atmosphere we know today. Instead, it harboured a reducing atmosphere with elements like hydrogen, methane, and ammonia. This environment was crucial for preventing the rapid oxidation of organic molecules, allowing them to accumulate and interact.
Right Chemicals: A combination of water, inorganic ions, and organic molecules was present. These building blocks were crucial for the formation of more complex compounds. Additionally, reactions involving electrical discharges, mimicking lightning strikes, likely formed some key molecules, such as amino acids
Energy Source: Energy for chemical reactions was provided by various sources, including UV radiation, electric discharges, volcanic activity, and cosmic rays. These energy sources facilitated the formation of complex molecules and promoted the transformation of simple compounds into more intricate ones.
Infinite Time: Over billions of years, these processes unfolded, giving life ample time to arise and evolve. The gradual accumulation of complex molecules, driven by the slow but persistent energy sources, allowed for the emergence of the first self-replicating systems.
The Steps of Life’s Emergence
According to this theory, the origin of life is divided into three steps.
Origin of Earth and Its Primitive Atmosphere:
Initially, Earth was a fiery ball of gases and vapours. Over millions of years, gases condensed into a molten core and elements stratified by density. Light elements like helium, hydrogen, oxygen, nitrogen, and carbon flowed to the surface, forming a gaseous atmosphere (different from today). Earth remained hot. As it cooled, some atmospheric gases liquefied, and others solidified. Steam condensed to water, causing rain – shaping the primitive atmosphere. This early Earth’s atmosphere was reducing, holding hydrogen, methane, ammonia, and water vapours. There was no free oxygen or ozone layer; UV rays reached Earth: UV rays, electric discharges from thunderclouds, volcanic heat, and cosmic rays fuelled reactions.
Chemical Evolution (Chemogeny)
Initially, the Earth’s extreme heat hindered the bonding of elements like H, O, C, and N. As the planet cooled, these free atoms began combining, forming simple compounds—nitrides, oxides, ammonia, methane, and cyanide. Energized by external sources, these compounds underwent reactions, producing more complex organic molecules.
Hydrocarbons were the earliest organics, followed by reactions that created more complex substances: esters, alcohols, ketones, acids, sugars, fatty acids, and amino acids. The sea, referred to as a ‘hot dilute soup’ by Haldane, likely fostered the synthesis of complex organic compounds. Here, simple molecules merged, reacted, and formed more complex molecules. Water and ammonia were among the initial compound molecules on early Earth. Methane was the first organic compound, followed by hydrogen cyanide. Protein molecules’ emergence marked a pivotal step in life’s origin.
In 1924, Oparin observed coacervates forming from a blend of proteins and polysaccharides. These contained proteins, polysaccharides, and water, displaying rudimentary metabolism. Oparin’s coacervates lacked lipid membranes, thus not meeting life precursor criteria. Mixing artificially produced organic compounds with cool water yielded microspheres. In 1950, Sydney Fox heated 18 amino acids, producing stable protein-like macromolecules called proteinoids. Cooling revealed small spherical units from proteinoid aggregations, termed proteinoid microspheres.
Earliest non-cellular life forms date back around 3 billion years, composed of giant molecules—RNA, proteins, and polysaccharides. Proteinoid microspheres, akin to coccoid bacteria in size, displayed spherical microscopic characteristics. Electron microscopy showed concentric double-layered boundaries aiding material diffusion. These microspheres exhibited motility, growth, binary fission, and reproduction via budding and fragmentation.
Experimental Validation
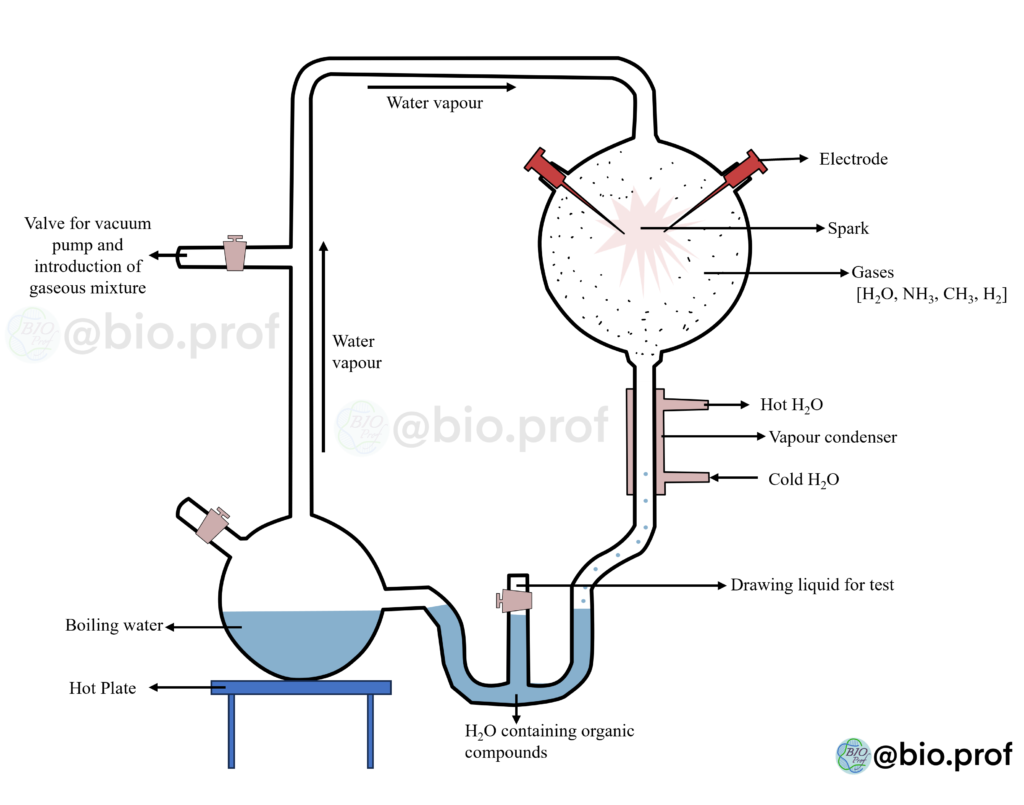
The majority accepts Oparin-Haldane’s theory regarding the chemical origin of life due to the supporting evidence from experiments. Among these experiments, the Miller-Urey experiment is particularly famous. This experiment demonstrated that simple organic compounds could naturally form, as proposed by Oparin-Haldane’s theory. In 1953, Stanley L. Miller and Harold C. Urey conducted a simulation experiment to showcase this concept.
They designed the spark discharge apparatus to replicate the conditions of the primitive Earth. This apparatus consisted of interconnected components, including a gas flask, a condenser, and a liquid flask. To facilitate the experiment, these components were equipped with sources of energy. The goal was to mimic the atmosphere of the early Earth, characterized by a “reducing atmosphere” (in the gas flask) and an “ocean” (in the liquid flask).
The experiment involved circulating a mixture of methane (CH4), ammonia (NH3), and hydrogen (H2) in a ratio of 2:1:2, along with water vapour (H2O), at a temperature of 800°C. Scientists believed that these conditions resembled those of the ancient atmosphere. Electric sparks generated by electrodes in the gas flask provided the necessary energy for the interaction among the gases in the mixture, simulating the role of lightning as a source of energy.
The experiment then condensed the gases in a narrow tube and passed them through the liquid flask. In this step, the experiment supplied energy as heat using an electric heater, mimicking the heat generated by a volcanic environment. Miller and Urey allowed the experiment to run continuously for 18 days. The experiment resulted in the formation of a mixture of small organic molecules in the gas flask. These molecules were then transported to the liquid flask through condensation, resembling rain falling into an ocean.
Subsequent analysis using techniques like chromatography and calorimetry confirmed the presence of various organic compounds, including amino acids like glycine, alanine, and aspartic acid, as well as adenine and simple sugars like ribose. These findings effectively validated Oparin-Haldane’s proposition that the conditions prevalent on early Earth could stimulate the transformation of inorganic compounds into simple organic ones, ultimately providing the foundation for the emergence of life.
Biological Evolution (Biogeny):
Due to aggregation, two primary types of giant nucleoprotein molecules emerged from the union of nucleic acid and proteinoid molecules. The first type encompasses nucleoproteinoid micro-particles, exhibiting either a fibrous or a globular appearance. These proto-ribosomes are hypothesized to have given rise to early ribosomes. The second type consists of colossal nucleoproteinoid molecules that exhibit certain characteristics akin to ‘free-living genes.’ We can draw a parallel between these molecules and present-day viruses, which possess nucleic acid cores encased in protein coatings. Oparin termed these entities ‘protoviruses‘ or ‘protobionts.‘
Origin of Prokaryotic:
Scientists postulate that the initial living organisms were chemoheterotrophs, deriving energy from the fermentation of complex organic substances present in the oceanic broth. Their rapid multiplication led to the depletion of nutrients in the seawater. Consequently, other nutritional modes such as parasitism, saprophytism, predation, and chemoautotrophism emerged. The cooling of the Earth’s temperature further hindered the synthesis of these nutrients, as their production rate was much slower than their consumption rate.
Chemoautotrophs, or organisms capable of chemosynthesis, emerged. They synthesized organic materials from inorganic compounds using energy from reduced inorganic substances in the environment. Modern-day examples include iron bacteria, nitrifying bacteria, and sulphur bacteria. Some protocells evolved into photosynthetic entities, incorporating chlorophyll and utilizing light energy for carbohydrate synthesis. These photoautotrophs were initially anaerobic, not reliant on water as a hydrogen source but rather cleaving hydrogen sulphide into hydrogen and sulphur. This yielded organic compounds as food and released sulphur as waste.
Gradually, anaerobic photoautotrophs transitioned into aerobic forms, facilitated by the accumulation of CO2 in the atmosphere and the development of chlorophyll molecules. The earliest aerobic photoautotrophs resembled cyanobacteria and used water as a hydrogen source and carbon dioxide as a carbon source for photosynthesis. This process releases oxygen into the atmosphere, making them oxygen-producing photosynthesizers. These entities emerged around 3.5 billion years ago, releasing oxygen into the sea and eventually the atmosphere. This atmospheric oxygen reacted with methane and ammonia, transforming them into CO2 and free N2, leading to the shift from a reducing to an oxidizing atmosphere.
Origin of Eukaryotic organism
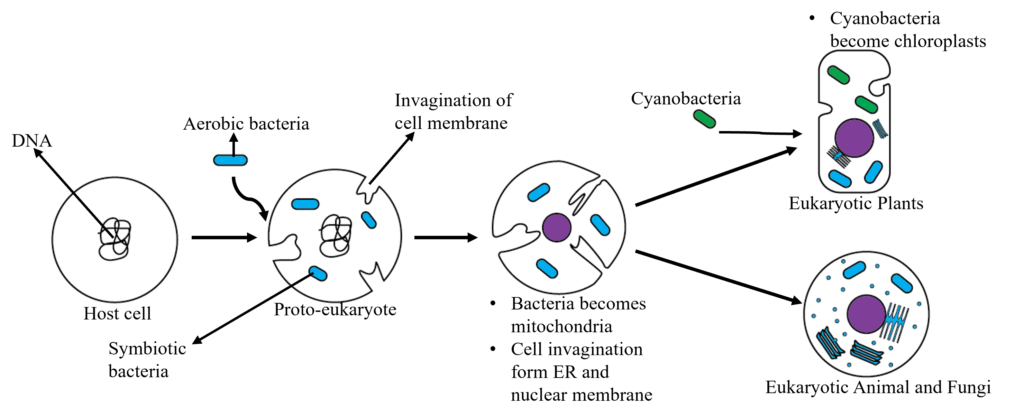
As oxygen levels rose, single-celled eukaryotic organisms emerged. These eukaryotic cells are believed to have evolved from archaea, which are prokaryotic cells. Two theories regarding their origin exist the symbiotic origin and the origin by invagination. The former proposes that anaerobic predator host cells engulfed primitive aerobic bacteria, establishing a mutual association and forming the first eukaryotic cells. The latter theory suggests that organelles within eukaryotic cells evolved through the invagination and separation of the surface membrane of primitive prokaryotic cells. Current scientific understanding suggest that eukaryotic cells evolved from prokaryotic cells through both processes, such as the nucleus evolving through invagination and mitochondria through endosymbiosis.
Regarding the origin of multicellularity, two pathways are postulated. One involves the formation of colonies by unicellular organisms to enhance coordinated functioning, subsequently leading to cell differentiation and multicellular organisms. The other route suggests that daughter cells of unicellular organisms failed to separate after division, resulting in an undifferentiated mass of cells that eventually specialized for specific tasks, forming multicellular organisms. The benefit of multicellularity lies in the division of labour among cells, leading to the organisation’s increased complexity. This gradual transformation of complex life forms from simpler ones is known as organic evolution.
Looking Ahead
The chemical theory of the origin of life challenges our understanding of life’s beginnings. It underscores the importance of the unique conditions on early Earth, where reducing atmospheres and energy sources facilitated the gradual assembly of complex molecules. While the theory plausibly explains life’s emergence, ongoing research and experimentation are still exploring the intricacies of this process. As we uncover more about the early Earth and the chemistry of life, we move closer to unravelling the mystery of life’s origin. This exploration sheds light on the past and deepens our understanding of the potential for life to arise elsewhere in the universe. The Chemical Theory of the Origin of Life reminds us of the remarkable journey from simple molecules to the diversity of life forms that populate our world today.

Pingback: MCQ: the chemical theory of the Origin of life - BioProf